
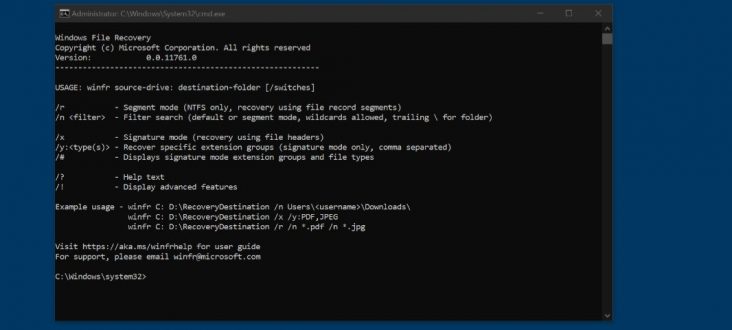
This dataset comes with a BBBC021_v1_image.csv, which is a CellProfiler CSV Data file. # Rerun this when you want to run the entire analysis # It will take some time, so don’t run until you’re sure! cellprofiler - run - run-headless \ -p illum.cppipe \ -o Week1/Week1_22123 -i Week1/Week1_22123 For just troubleshooting and thinking about how you want to batch your analysis, you are fine just running the first image set. When you want to run the entire pipeline as a whole you should rerun the illum.cpipe with no -f or -l. Because the Illumination Pipeline computes some average illumination files, we shouldn’t use the divide and conquer approach. You should see some output that looks like this: Let’s make sure that we can process at least the first image. We will not actually use this file, but it is useful to understand the structure of the analysis. cd /project/BBBC021 head -n 1 BBBC021_v1_image.csv > images_week1.csv cat BBBC021_v1_image.csv | grep Week1_ > images_week1.csv We’re only using Week 1 because I’m doing this on my laptop. The data file that comes with the set includes data for all weeks.
The analysis pipeline seems to expect that the output from the illumination pipeline exists in the same directory. You should have images in the illum_corrected, labels and overlay directories, and csv files in the measurements directory. I had to play around with the exact inputs and outputs to get this to work without errors, but how it works is: illum.cppipe Week1/Week1_22123/ # Inputs to the Illumination AND Analysis Pipeline Week1*.tif # Outputs to the Illumination Pipeline # Inputs to the Analysis Pipeline Week1_22123_Illumctin.npy Week1_222123_IllumActinAvg.npy Week1_222123_IllumDAPI.npy Week1_222123_IllumDAPIAvg.npy Week1_222123_IllumTubulin.npy Week1_222123_IllumTubulinAvg.npy # Outputs to the Analysis Pipeline overlay labels measurements illum_corrected
You will also see that there are 2 pipelines, an Illumination correction pipeline and then an Analysis pipeline. I think it’s best explained from the dataset itself. You should be in /project/BBBC021 wget wget wget wget wget Then create a file called Dockerfile with this: FROM cellprofiler/cellprofiler:3.1.9 RUN apt-get update -y apt-get install -y unzip imagemagick ENV TINI_VERSION v0.16.1 ADD $ cd. mkdir cellprofiler-batch-tutorial cd cellprofiler-batch-tutorial We are making these changes because the image is set up in such a way that it is very well suited for a job queue environment, but what we want here is to dig around with some exploratory analysis.Ĭreate a project directory, cellprofiler-batch-tutorial, and cd into it. We will be using the default CellProfiler docker image with a few changes. Here’s a quick explanation and tutorial from Ubuntu to get you started. This does not require too much Linux command line knowledge, but you will need to be able to type commands and navigate a directory structure. Quick disclaimer, if you are very uncomfortable with the command line you may want to reach out for help. Once you have it all set up you treat it just as you would a regular computer. A docker container is like a virtual machine, except without a visual interface. If you prefer to watch, here is a video where I go through the steps described.ĭocker is a way of packaging applications.

Here’s a tutorial of step 7, submit your batches to the cluster. The instructions for the CreateBatchFiles module describe how to set up a CellProfiler pipeline and submit it to a cluster. If you need to find a way to decrease your computational resources you can often split your dataset. The larger the dataset you are analyzing the more memory it consumes. This is also an important consideration if you are running out of memory or CPU. You could split that analysis into 4 chunks, and each would complete in 15 minutes. Say you have a large dataset that would take 1 hour to complete. This is helpful when you have a very large dataset. One of the benefits of running CellProfiler in batch is that you can split your analysis. Or not so large scale analyses that you prefer to automate! Running CellProfiler in batch mode is the ideal way to automate large scale analyses.


 0 kommentar(er)
0 kommentar(er)
